Don't knock it
 We live in interesting and uncertain times - that much at least is certain. The world may be hovering on the point of economic disaster but in 2011, despite the proliferation of other types of fuel (such as ethanol, methanol, CNG or diesel) the performance fuel of choice is still gasoline. A mixture of up to several hundred types of hydrocarbon chains of varying length and shape, gasoline can be tailored more specifically to the precise characteristics of an internal combustion engine than perhaps any other fossil fuel. And of its many properties the two most important are volatility and resistance to self-ignition.
We live in interesting and uncertain times - that much at least is certain. The world may be hovering on the point of economic disaster but in 2011, despite the proliferation of other types of fuel (such as ethanol, methanol, CNG or diesel) the performance fuel of choice is still gasoline. A mixture of up to several hundred types of hydrocarbon chains of varying length and shape, gasoline can be tailored more specifically to the precise characteristics of an internal combustion engine than perhaps any other fossil fuel. And of its many properties the two most important are volatility and resistance to self-ignition.
For track-based vehicles where ease of cold starting and fuel economy is not that critical, the volatility of the fuel is of secondary significance. Race fuels consequently tend to have much flatter volatility curves than road transport or forecourt fuels, generally reflecting the increased content of individual hydrocarbon compounds within them. So while it may be difficult to start the engine from cold, at the other end of the temperature range the high level of mid-range volatility creates difficulties with hot restarts as the fuel vapour collects in the fuel lines. A minor inconvenience if nothing else, but by far the most critical performance attribute is the fuel's resistance to self-ignition or detonation - more commonly known as 'knock.'
Described in some textbooks as 'the noise transmitted through the engine structure caused by the spontaneous combustion of the fuel/air end-gas in advance of the combustion flame front', the fuel characteristic that best resists this is referred to as its octane. A rather arbitrary measure on a scale of 0 to 100, where 0 is the performance using n-heptane and 100 is that for iso-octane, the octane requirement of an engine varies depending on things like speed, charge temperature, combustion chamber geometry, compression ratio and engine operating conditions - not forgetting, of course, the time available at the appropriate conditions for the events to occur.
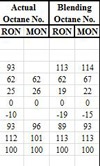
Governed by these engine factors the presence or absence of knock will eventually be down to the qualities of the fuel. So while they are made up of several hundred individual hydrocarbon species, each one with its own volatility or boiling point, each component also has its own ability to resist combustion knock. But while an individual compound on its own may exhibit one octane number, when blended into the fuel it will act as if it had a totally different octane value. This value is called the blending number, and often serves only to confuse.
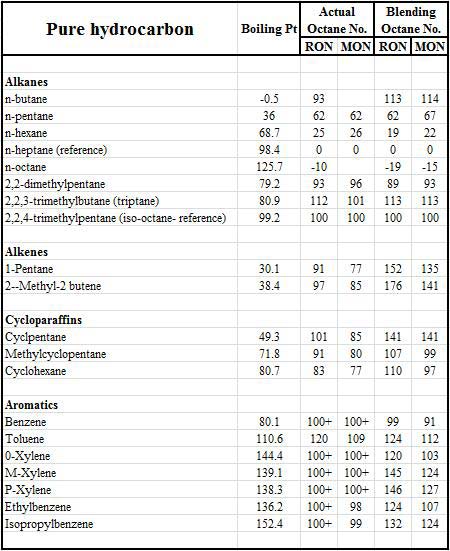
On top of this, simply changing the position of one of the methyl groups in a hydrocarbon molecule - keeping the same number of hydrogen and carbon atoms and the same number and type of covalent bonds - can also have a significant effect. Decreasing the length of a simple hydrocarbon chain and incorporating a methyl group as a side chain has the effect of improving the octane. For instance, normal, straight-chained octane (n-octane) has a boiling point of 126 C but blends into the fuel as if its octane was -17. On the other hand, the branched chain iso-octane, with the same chemical formula and a slightly lower boiling point of 99 C, has an octane of 100. In general, reducing the number of carbon atoms in the main chain reduces the tendency to knock, but at the same time reduces the component boiling point, and so increasing its volatility.
The task of the fuel blender is therefore to take all these properties (and others) to create the correct 'balance' for your engine. And if he (or she) can do that successfully then why not give them the task of sorting out the global economy? Surely they can't do any worse than our politicians.
Fig. 1 - Comparison of actual octane and blending octane for some of the more common fuel hydrocarbons
Written by John Coxon