Ethanol and water - and disappearing octane!
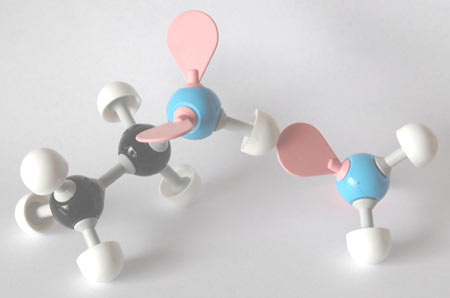
 It could be that I'm giving the wrong impression here but on a cold winter's evening there is nothing I like so much as a small glass of whisky. Settling into my favourite armchair alongside the dying embers of a log fire and savouring the delights to come, I watch carefully as the dash of tepid water (not too much now!) is added to the tumbler and then slowly mixes. That it does so is one of life's extraordinary things, as the surplus electrons of the water's oxygen atoms are attracted to the electron-deficient hydrogen atoms of the alcohol, strangely boosting the flavour and enhancing the delights of my evening ritual. And while I am happy to accept these - the hydrogen bonds in my whisky - when mixed with motor fuel the presence of water in the ethanol is not always quite so comforting.
It could be that I'm giving the wrong impression here but on a cold winter's evening there is nothing I like so much as a small glass of whisky. Settling into my favourite armchair alongside the dying embers of a log fire and savouring the delights to come, I watch carefully as the dash of tepid water (not too much now!) is added to the tumbler and then slowly mixes. That it does so is one of life's extraordinary things, as the surplus electrons of the water's oxygen atoms are attracted to the electron-deficient hydrogen atoms of the alcohol, strangely boosting the flavour and enhancing the delights of my evening ritual. And while I am happy to accept these - the hydrogen bonds in my whisky - when mixed with motor fuel the presence of water in the ethanol is not always quite so comforting.
The fact that water and ethanol are fully miscible in each other with no limits is by now I hope evident to all, and naturally therefore, when exposed to the humidity of the atmosphere, such water will inevitably migrate into the alcohol without limit. When high levels of ethanol are used, for example E100 (100% ethanol) or E85 (85% ethanol), the presence of comparatively small amounts of water can be tolerated - indeed, industrial-quality ethanol can have as much as 4.9% water in it simply as a result of the manufacturing process and the energy required to refine the product to a anhydrous higher level.
But when only small amounts of the stuff are added to the likes of E10 or E15 - as in the case of many modern-day gasoline blends or when the octane-enhancing benefits of MTBE (methyl tertiary butyl ether) have been replaced by less polluting ethanol - the presence of water can be a problem. Let me explain.
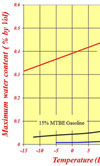
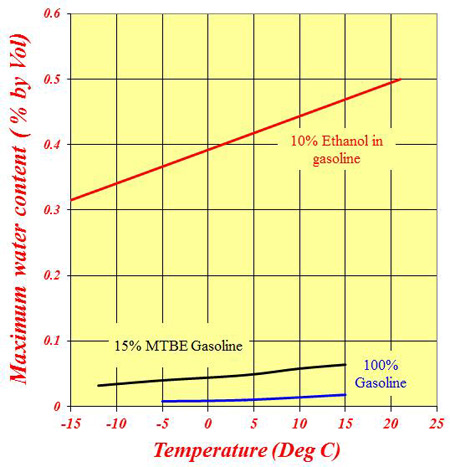
For all practical purposes, while water is fully miscible in ethanol, it is totally immiscible in gasoline. That's not quite true since most gasoline will have a very small amount of water in it - maybe 0.01% or 0.02% by volume - but, by and large, water will not dissolve in the fuel. In a fuel tank full of gasoline, once the water has exceeded this level the surplus water will fall to the bottom of the tank and hopefully out of harm's way. In the presence of an oxygenate such as MTBE, the maximum amount (of water) absorbed can increase slightly to about 0.06-0.07% at ambient temperature, and any excess will fall to the bottom of the tank again.
However, if this oxygenate is replaced by a less polluting ethanol, the mixture will quite readily absorb as much as 0.4-0.5% of water, and since ethanol is highly hygroscopic, it will do so. As a guide, a drum of fuel left unsealed in an atmosphere of 70% relative humidity will absorb this amount in three months. Once the water exceeds this level, 'phase separation' occurs and the water-ethanol mixture, not just the water, will sink to the bottom of the tank, effectively denuding the gasoline of its octane. The more water that is absorbed, the less octane in the remaining fuel. I'll leave you to imagine the rest.
So while a smidgeon of water may enhance your drinking pleasure, in your fuel tank it can create so many more issues with your engine.
Fig. 1 - Variation of the maximum water absorbed in fuels with temperature
Fig. 2 - Water - good or bad? Model of the hydrogen bond: the 'spare' electrons in the outer shell of the water oxygen atom (in pink) are attracted to the electron-deficient hydrogen molecules of the alcohol (in white)
Written by John Coxon