The Greening of Formula One
 It seems ironic to think that amid the hype of the introduction of kinetic energy recycling systems (KERS) for the 2009 season, the real ‘greening’ of our premier racing category started precisely one year before. At that time the ‘green’ debate was at its height and faced with EU legislation covering road fuels for 2010, the FIA unilaterally introduced the ruling that for 2008 a minimum of 5.75% by mass of the fuel must comprise of oxygenates ‘derived from biological sources’. The move was designed to head off criticism of the sport’s wasteful ways and to pre-empt the EU’s somewhat controversial introduction of EU Directive 2003/30/EG.
It seems ironic to think that amid the hype of the introduction of kinetic energy recycling systems (KERS) for the 2009 season, the real ‘greening’ of our premier racing category started precisely one year before. At that time the ‘green’ debate was at its height and faced with EU legislation covering road fuels for 2010, the FIA unilaterally introduced the ruling that for 2008 a minimum of 5.75% by mass of the fuel must comprise of oxygenates ‘derived from biological sources’. The move was designed to head off criticism of the sport’s wasteful ways and to pre-empt the EU’s somewhat controversial introduction of EU Directive 2003/30/EG.
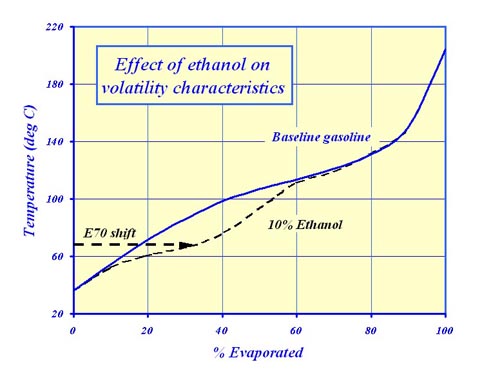
However, this was carried out with very little fanfare, and few even knew about it.Oxygenates, no matter what their source, are good for anti-knock behaviour and hence power, and have been widely used in pump fuels ever since the earlier anti-knock additive, tetraethyl lead (TEL) was phased out in the 1990s. Initially introduced in the form of MTBE (methyl tertiary butyl ether) – CH3.O.C4H9 – in response to US and now EU legislation, they are now being replaced by their bio-derived counterparts.The high octane, bio-oxygenates of most interest are ethanol and ETBE (ethyl tertiary butyl ether). Whereas MBTE was produced from fossil fuel, ETBE is manufactured from ethanol, which itself comes from bio sources and therefore ETBE can claim to have some ‘green’ credentials. However, it is the blending of ethanol into gasoline that raises a number of issues.The first of these is that the addition of only a very small amount of ethanol (vapour pressure – 16 kPa) to a gasoline mix (maximum vapour pressure - 60 kPa) actually increases the vapour pressure of the overall fuel. Explained by the formation of hydrogen bonds, when using small quantities of ethanol, this is contrary to what one might intuitively think. Thus for our hydrocarbon fuel of say, 60 kPa vapour pressure, the introduction of 2% ethanol will increase the vapour pressure to 67.3 kPa. At 5% the vapour pressure will increase still further to a maximum of just over 68 kPa. After this, the vapour pressure will begin to fall again as this hydrogen bond effect is overtaken by the reduced vapour pressure of the additive such that at 5.75%, the maximum stipulated by the FIA, the mixture will fall to 68 kPa. Thus a fuel, which was initially within the limits in the rules (45.0 – 60.0 kPa), could now be illegal once ethanol is added.However, unlike EN228, the legal requirement for forecourt fuels, Article 19 of the Formula One regulations does take this into account allowing a maximum of 680 hPa vapour pressure when a minimum of 2% or more of bio-ethanol is used.Another issue in the use of ethanol is that it distorts the volatility curve. In particular, it pushes up the E70 value (the percentage of the fuel evaporated at 70 degrees C) and means that careful re-blending with a low volatility blend stock is necessary. Other than pushing the fuel outside of the designated bands, this is not a major issue within Formula One. But for commercial fuels, problems with cold starting and drivability could be encountered unless this is corrected at the blending stage.
Another major issue with ethanol, perhaps not widely publicised, is its affinity for water. Most could not have failed to notice that water mixes ever so well with your favourite alcoholic tipple, well, the same applies to ethanol when mixed into gasoline. Water in very small amounts will almost inevitably be found in any liquid fuels made on an industrial scale. In the case of gasoline, depending on its temperature and composition, this water will dissolve only slightly (less than 0.01%) and the rest will accumulate at the bottom of the tank. When mixed with ethanol, however, the water is completely miscible and may cause the ethanol-gasoline blend to separate out with an alcohol-enriched water layer at the bottom of the tank effectively denuding the gasoline–ethanol mix above it. Again not a problem with Formula One fuels, when contamination can be eliminated with careful handling and storage, but for commercial forecourt fuels the risk is ever present.Thus while the teams struggle to develop their so-called ‘green’ KERS systems, the fuel suppliers just quietly get on with the job.