Compressed natural gas
 Many years ago, journalists and vehicle road testers used to talk about 'cooking engines'. At the time I understood it to be a term describing the performance of the unit, but I was unsure if it related to a high-performance, highly tuned engine or simply a fairly standard, docile unit reminiscent of a kitchen stove; the term meant nothing to me. Today, of course, with increasing awareness of fuel security and global warming, any engine described as 'cooking' must surely be the latter, and fuelled by the one fuel which is distributed about the land straight into our kitchens - natural gas.
Many years ago, journalists and vehicle road testers used to talk about 'cooking engines'. At the time I understood it to be a term describing the performance of the unit, but I was unsure if it related to a high-performance, highly tuned engine or simply a fairly standard, docile unit reminiscent of a kitchen stove; the term meant nothing to me. Today, of course, with increasing awareness of fuel security and global warming, any engine described as 'cooking' must surely be the latter, and fuelled by the one fuel which is distributed about the land straight into our kitchens - natural gas.
Consisting primarily of methane and smaller quantities of the higher saturates (ethane, butane and so on) together with 'inerts' (mainly nitrogen but also carbon dioxide), natural gas is found in many parts of the world deep underground. In its raw state the gas may also contain water, hydrogen sulphide (H2S) and some of the higher hydrocarbons, but these have to be removed before transportation to prevent condensation at the higher pressures or to avoid corrosion in the pipeline.
When using the fuel in a gas cooker the major concern is that of the gases' Wobbe Index. Defined as the volumetric heating value of the gas divided by the square of its density, natural gas is sold to the domestic user on this basis. Used in an engine, however, this is not quite so relevant. For an engine our main concern is octane requirement or, more specifically, the amount of compression the air-gas mixture can withstand before spontaneous ignition.
While the octane of the primary constituent, methane, is extremely high (around 130 RON, 122 MON) those of the possible contaminants (ethane, propane and butane) can be considerably less. The Motor Octane number for ethane is 101, while those for propane and butane are 96 and 89 respectively. Consequently, for use in an engine, the purer the methane the better.
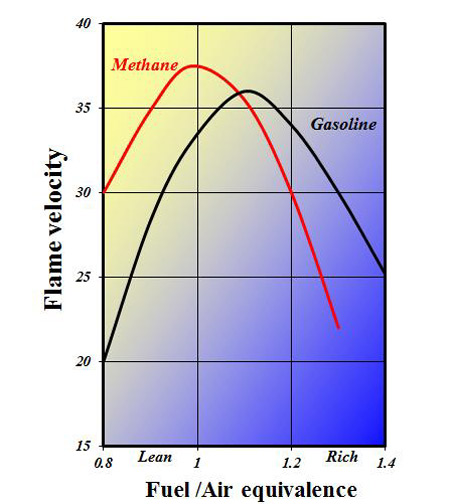
Even in its purest form, however, natural gas comprising almost 100% methane still has other issues, two of which are its laminar flame speed and the comparatively poor lean flammability. The fact that methane can be readily seen bubbling up through rotting vegetation in ponds and reed beds suggests it is a fairly stable molecule, not readily reacting chemically with other components in the natural world. By the same token it is difficult to ignite, and even when finally burning in the combustion chamber of an engine its flame speed can at times be appreciably slower than gasoline, particularly when running rich. So while its anti-knock potential is great, the potential to extract that performance is compromised by its burning characteristics.
Hydrogen, on the other hand, has characteristics that are almost complementary in that it has a much wider flammability range and is easily ignited. When added to methane in small amounts (up to as much as 5-7%) therefore, it greatly assists the ignition characteristics. Furthermore, with a flame speed of up to eight times higher than methane, once ignited - and particularly in the initial 0-10% burn period - the fuel-air mixture will burn quicker and more completely.
Referred to as HCNG or in some circumstances as Hythane, the use of such mixtures could eventually bring us one step closer to that other Utopia - that of the hydrogen economy.
Fig. 1 - Laminar flame velocity of methane compared to a typical gasoline mixture
Written by John Coxon