E10 - the fuel of the devil?
There are few issues likely to get owners of classic and vintage vehicles in the UK hot under the collar than changes to the composition of the fuels. Fifteen or maybe more years ago, the furore was all about lead. Added to petrol at 0.15 g/litre, not only did it supply cheap octane to the fuel by improving combustion by avoiding detonation but the tetraethyl lead compounds used also coated valve seats, reducing wear and helping to minimise valve seat recession.
Complicated by the phasing out of four-star 97 RON fuel and replacing it with a 95 RON - so-called 'premium' unleaded - the fuel companies produced a 98 RON lead replacement fuel (LRP) that replaced the octane and trusted enthusiasts to fit hardened valve seats to contain the wear. And when sales of the more expensive LRP fuel decayed a few years later, the compromise fuel was quietly dropped from the forecourt. By then specialist fuels were on the market in small amounts for owners who absolutely needed them, and those remaining used approved lead replacement additives.
These days, however, the issue is one of ethanol, and while the lead issue was all about the IQ levels of children living close to urban motorways, now the issue is one of sustainability and environmental considerations - and a UK government initiative called the Renewable Transport Fuel Obligation. The current standard for UK road fuel, BS EN 228, allows for up to a maximum of 5% of ethanol by volume. In practice, according to the individual refinery and the issues surrounding the transportation of the hygroscopic (water-absorbing) ethanol, not all fuels will contain this level, and fuel sold will contain either no ethanol or about 4-5 % depending on where you live. At this level there is no requirement to identify the ethanol content, and in most cases the general level of the fuel's performance is unchanged in any case.
However, with the advent of the RTFO by some now unspecified time in the future (at one time rumoured to be 2013 but now refuted by the Department for Transport and Rural Affairs) this maximum content is to increase to 10% by volume. And if 5% didn't exhibit any truly discernable difference then 10% most probably will. For modern vehicles with fuel injection and adaptive control designed for modern fuels there are no issues, but for those with carburettors (remember them?) many pre-2000 vehicles and even early direct-injection designs, the problems could be significant.
These issues fall into three main categories - combustion, corrosion and compatibility.
Compared to the value of 14.7:1 for non-ethanol, 'normal' fuels, E10 (as it is now called) has a stoichiometric value of 14.1:1. If the specific energies are broadly the same while the performance given by each will be similar, in those vehicles using carburettors, engines will run about 4-5% lean. For fuel economy this might be acceptable, but if mixture strengths are not adjusted to compensate, valve seat burning could result. The greater volatility of the increased amount of ethanol could also introduce fuel vapour lock in the under-bonnet fuel lines, so these will also have to be routed away from sources of heat.
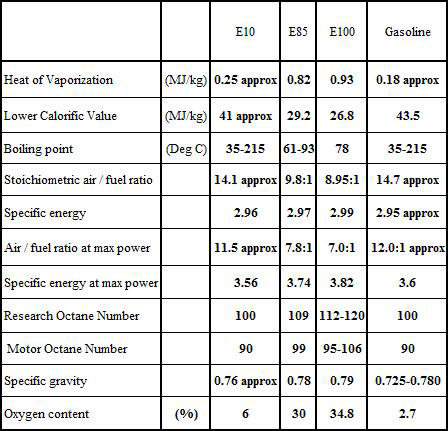
It has long been appreciated that ethanol-gasoline blends encourage corrosion when exposed to aluminium. The precise mechanisms involved, however, are complicated. Ethanol would seem to be a better conductor of electricity than hydrocarbon fuels, and the galvanic effect of dissimilar metals in the presence of absorbed water may have much to do with it. For racers the answer must always be to empty the fuel tank and run the engine on neat gasoline at the end of the day, but where this is impractical, galvanic action as a result of the proximity of dissimilar metals (such as aluminium-steel, aluminium-brass or zinc-brass) has to be avoided.
Elastomer compatibility also has to be taken very seriously when using the different fuel blends. Seal swell characteristics or the progressive hardening of elastomers can lead to system leaks, and it appears that, as in the case of corrosion, ethanol-gasoline blends are even more aggressive than either pure gasoline or pure ethanol alone. In general, however, the later types of fluorinated seal compounds are more resistant to attack than non-fluorinated types, and the greater the degree of fluorine then the higher the resistance.
In modern fuel systems these compatibility issues will all have been sorted, but being 'green' and protecting your fuel system for those with historic or classic vehicles may not be that simple.
Fig. 1 - Comparison of fuel characteristics
Written by John Coxon