Fuel economy
 To many, the words 'fuel' and 'economy' have no real place in the motorsports world. After all, and as everyone knows, to save fuel the driver has to be delicate on the throttle, avoid braking and keep in as high a gear as he (or she) can. And with these actions seemingly totally at odds with the concept of travelling quickly, I might find it hard to do anything other than agree.
To many, the words 'fuel' and 'economy' have no real place in the motorsports world. After all, and as everyone knows, to save fuel the driver has to be delicate on the throttle, avoid braking and keep in as high a gear as he (or she) can. And with these actions seemingly totally at odds with the concept of travelling quickly, I might find it hard to do anything other than agree.
Nevertheless, there are times when race organisers wish to restrict the amount of fuel carried on board and therefore stipulate a maximum tank capacity to which all competitors must comply. In such cases, in order to finish the race and assuming refuelling is not allowed (or even desirable), it will be necessary to eke out the fuel supply in some way or another.
The most obvious way is to trim back on the fuel map and instead of, say, running at 12:1 air-fuel ratio, nudge it back a little to 12.5:1 for example. Little power will be lost and, with luck, you may make it to the end of the race. At the same time, however, you may finish last!
But there is more than one way to obtain fuel economy. Take the blend of fuel used, for instance. A typical gasoline fuel used for motorsports consists of up to 200 or more individual hydrocarbon species, each of which has its own heat of combustion, octane rating, heat of vaporisation and specific gravity. Furthermore, each of these compounds - whether it is a member of the paraffin, olefin, cycloparaffin or aromatic group - will have a temperature at which it boils such that the whole mixture we call gasoline will have a volatility (or boiling point) curve similar to the one shown in the figure here.
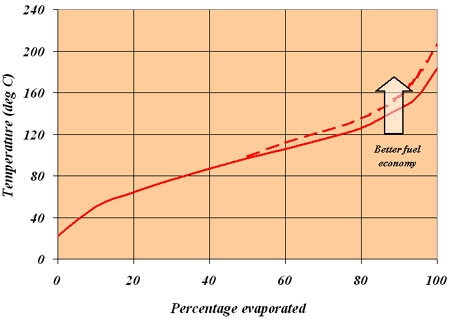
For most fuels this is in the form of a gentle S-shaped curve, which rises progressively from the left-hand side to a maximum at the right-hand side. Once fully blended, this volatility curve is a design attribute of the fuel, with the smaller and lighter hydrocarbons boiling off towards the lower portion of the curve and the heavier, longer-chain molecules towards the top end. At the lower end of this curve, if the volatility is too high, the engine may be difficult to start when cold; if it's too low then the vapour produced in the fuel line can make restarting when hot almost impossible. At the higher end of the curve, the idea of fuel economy introduces itself.
At this higher end, and with everything else being equal, moving to hydrocarbon components with a greater density will pack more heat energy into a given volume. In practice, the heavier hydrocarbons from the aromatics group with the generalised formula CnH2n-6 are used and, so long as the overall heat of energy and octane rating aren't unduly affected, will enable the engine to run slightly leaner.
Of course, it is important to ensure that the overall fuel density doesn't exceed that specified or that the overall volatility characteristic expressed in the RVP (Reid Vapour Pressure) becomes too low - or indeed that one of any number of constraints put upon the fuel is not transgressed. With this level of complexity it is easy to see why gasoline fuel blends can become so complicated and so different between suppliers.
The real skill in fuel blending therefore is to balance the volatility curve to suit the characteristics of the engine and yet still keep within the fuel regulations.
And in the hunt for fuel economy I would start with the fuel.
Fig. 1 - A typical gasoline volatility curve
Written by John Coxon