Nitriding
 Nitrogen is a colourless, tasteless, odourless, mainly diatomic gas that makes up about 78% of the air we breathe. Chemically it's almost inert, and as well as being a critical part of human DNA it can also often be an essential part of the DNA make-up of a piston ring. For example, titanium nitride and chromium nitride are popular piston ring coatings. Applied to the bore-contacting outer surface of steel rings, such surfaces are intended to reduce engine friction and reduce wear, but not to offer any additional strength to the ring.
Nitrogen is a colourless, tasteless, odourless, mainly diatomic gas that makes up about 78% of the air we breathe. Chemically it's almost inert, and as well as being a critical part of human DNA it can also often be an essential part of the DNA make-up of a piston ring. For example, titanium nitride and chromium nitride are popular piston ring coatings. Applied to the bore-contacting outer surface of steel rings, such surfaces are intended to reduce engine friction and reduce wear, but not to offer any additional strength to the ring.
All steels of course contain some level of nitrogen within them, particularly when in the molten state. With a maximum solubility of around 450 ppm, which drops to nearer 10 ppm once the material has cooled to ambient temperature, nitrogen can have a beneficial effect on steel if it remains in solid solution or precipitates out as a fine coherent nitride at the gain boundary. The presence of molybdenum, chromium or vanadium in the mix can also help the material form metal nitrides. However, as the amount of nitrogen increases, its degree of formability decreases, so it is often better to make the component first, introducing nitrogen into the surface layer at a later time.
Such a process is called nitriding, and is the accepted practise of forcing nitrogen into the surface of a steel component to produce a much harder layer which is more wear resistant. Typically somewhere around 0.001 in ( 0.025 mm) thick, this layer is comparable with other forms of case hardening, and gives a hard outer layer around a softer but tougher central core. Since lower temperatures are generally used, less distortion of the final product is to be expected. Processed after heat treatment and subsequent tempering, in some cases final grinding may be necessary to remove a hard 'white' layer before finishing to size.
More commonly associated with the case hardening of crankshafts and camshafts, there are principally three methods in use. The first is a salt bath that uses cyanide-based salts, and because of that it is no longer in general use. Of the other two methods - gas nitriding and ion or plasma nitriding - both are of potential interest to piston ring manufacturers. Gas nitriding, whereby components are placed in an atmosphere of nitrogen-enriched ammonia gas in an air-tight furnace, will produce all-over surface benefits. Plasma nitriding, however, as a result of its better targeting, can be confined to particular surfaces.
Used in diesel and other automotive OEM engines to replace the toxicity of chromium plating methods, nitrided rings can be used on their own against just about any Nikasil or iron bore surface and in the aggressive environments offered by exhaust gas recirculation. At around 110 Vickers harness (50% greater than the base steel) it has also been shown to offer improved durability over chrome. For competition use, nitrided rings have been shown to produce a higher level of performance over the traditional moly-coated alternative, since the nitriding layer is integral with the base steel and not an 'add-on' coating. In some circumstances molybdenum coatings have been known to flake off as a result of thermal shock experienced in heavily turbocharged engines.
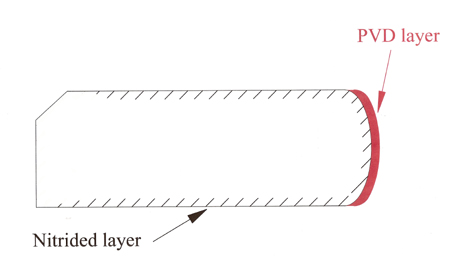
Mindful of the strength benefits that nitriding gives to the base ring material, in some cases manufacturers offer traditional PVD coatings on top of the nitrided layer - a sort of 'belt and braces' approach that produces even higher hardness numbers, with 1400-2200 Vickers being quoted.
Nitrogen may be all around and even within us, but when introduced to the surface layer of a steel piston ring its effects may be more noticeable.
Fig. 1 - A PVD-coated ring after nitriding
Written by John Coxon