Protect and Survive
 There was a time when simply using the correct grade of oil for your engine and changing it regularly would be all that was needed. Back in the days of the nuclear family, life was simple and we knew that our cam’s lobes were protected even if a nuclear war was potentially just around the corner at ‘five minutes to midnight’. Approaching the second decade of the 21st century and while the prospect of nuclear war has almost disappeared, the threat to some of our camshafts has assumed a level unacceptable in the modern world. And as ever, this has all been achieved under the guise of ‘saving the planet’.
There was a time when simply using the correct grade of oil for your engine and changing it regularly would be all that was needed. Back in the days of the nuclear family, life was simple and we knew that our cam’s lobes were protected even if a nuclear war was potentially just around the corner at ‘five minutes to midnight’. Approaching the second decade of the 21st century and while the prospect of nuclear war has almost disappeared, the threat to some of our camshafts has assumed a level unacceptable in the modern world. And as ever, this has all been achieved under the guise of ‘saving the planet’.
The tale is a sad one but one completely avoidable once you understand the situation. In recent years the oil designed for passenger cars has changed in formulation. In response to the need to reduce precious metal loadings in modern vehicle catalysts and at the same time increase their durability, certain key components have had to be reduced or replaced in the latest generation of PCEOs (Passenger Car Engine Oils). Critically for gasoline engines, these key compounds are those containing sulphur and phosphorus which rather regretfully are the same components found in one of the most effective anti-wear oil additives commonly in use, that of ZDDP, or zinc dialkyldithiophosphate.
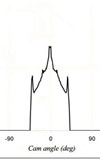
In any crankcase engine oil the formulation has to contain certain anti wear additives to protect the surface of the engine components when the relative motion of the mating parts falls below a certain threshold level. At this point, should no other means of protecting the surface be available, there is a great risk that the oil film is insufficiently thick to prevent surface to surface contact when hydrodynamic lubrication progressively falls back into the boundary type. Metal to metal contact will take place and result in friction, high temperatures and ultimately the failure of the parts. Of all the components in an engine, the cam - flat tappet interface, whether in the form of the direct acting mechanical bucket in overhead cam engines or to a slightly lesser extent mushroom tappets in pushrod engines, is that most at risk. In these circumstances the oil entrainment velocity, the relative velocity of the cam-tappet contact point on the tappet with respect to the velocity of that point on the cam, drops to zero and then reverses at slow speed. If this relative velocity is insufficient to generate a ‘wedge’ of oil between the two, then these surfaces will rely on the presence of the highly polar ZDDP molecules to keep the surfaces apart and protect them.
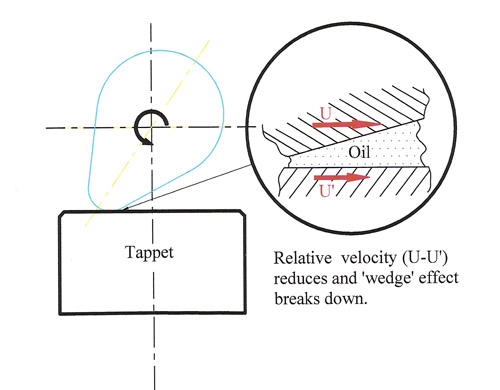
Up until recently typical zinc levels of 1200 ppm were sufficient to protect the flat tappet and once the temperature of the oil was above 55-60 deg C, these components would become highly surface active, attracting themselves to the nearest metal surface. Thus while the viscosity of the oil decreased these additives would protect all metal surfaces and enhance the property of the lubricant as the temperature increased. Come the arrival of ‘Emission System Protection’ oils in Europe (ACEA C1- C3) and GF-4 fuel economy oils in the US, the level of zinc has been regulated to much lower levels of 500-900 ppm depending upon the viscosity grade. And while other forms of anti-wear have been developed they are not necessarily as effective nor perhaps rather more importantly as cheap as their earlier counterparts. Designed for and proved on the more modern, low friction valve trains of the latest generation of passenger cars, these low zinc, phosphorus and sulphur oils should not be used on older flat tappet engines.
While in the UK the ‘Protect and Survive’ booklet of the 1970s was somewhat fatalistic in the event of nuclear war, the current threat to our older flat tappet valve train is easily parried by using the appropriate engine crankcase oil for the task. In general most oils of the right grade for your engine and designed specifically for competition will satisfy this requirement.
Written by John Coxon