Formula One fuel the turbo years
1988 was a memorable year. Not only were the two greatest drivers of their time – Ayton Senna and Alain Prost – battling for supremacy, and doing it within the same team in similar cars, it was also the last time turbochargers were used in anger in Formula One.
Back then, and much like 2014 in some respects, one team dominated. In 1988 it was McLaren. Winning all but one of the races that year using a V6-configured engine; there the similarities end though. In 1988, the winning engines (the Honda 1.5 litre RA168E, for thus it was designated) were reputed to deliver something like 504 kW (685 PS) at 12,500 rpm on 2.5 bar boost using a fuel consisting of 84% toluene and 16% normal heptane. Of course, regulation in Formula One was much less restrictive back then, and so long as the fuel used bore some resemblance to road-based or ‘pump’ fuels – in that it had a Research Octane Number (RON) no greater than 102 (the Honda fuel was 101.8) – then all was well. The lack of low boiling point constituents in some fuels, which meant they had to be heated to around 75 C before being injected into the engine, wasn’t seen as an issue, and neither was the reportedly evil-smelling brews of other fuels, but hey, this was the 1980s and Formula One was pushing the boundaries.
In 2014, the engines could appear to the uninitiated to be very similar to those used in 1988. These days we still have V6 engines – this time 1.6 litres – and we now have turbochargers again, a single unit as opposed to most teams using twin units in ’88, but the ‘forecourt’ fuel of 2014 is totally different from that used in 1988. Indeed, even though Article 19 of the technical regulations remains unchanged the fuel used in 2014 will be significantly different from that used in 2013.
In designing a fuel for any engine, fuel technologists will look at many aspects. The heating value, the stoichiometry and even the density of the fuel in some cases will all be considered. But the fuel requirements of a 2.4 litre naturally aspirated V8 revving to 18,000 rpm will be totally different from those of a 1.6 litre turbocharged V6 doing not much more than 12,000 rpm. So from 2013 to 2014, although the fuel regulations did not change so far as the make-up the fuel is concerned, the precise blend of hydrocarbons within it almost certainly will have.
At 18,000 rpm the fuel has about 0.001 s in which to burn from the point of ignition to the opening of the exhaust valve. As it burns progressively across the bore, if the fuel ignites in front of the flame front then detonation will occur. This will cause a sudden increase in local pressure and temperature in the cylinder which, if allowed to continue, will damage the piston. At 18,000 rpm the speed of burn and the motion within the cylinder is such that there is generally insufficient time for this to occur. At this speed, this resistance to detonation (or ‘knock’) governed by the fuel’s octane number is therefore not so important.
However, at 12,000 rpm – the typical top speed of the new V6 engines –the time available for this flame front to move across the bore is now 50% greater, which is a lot more time for any stray pockets of end gas from previous combustion cycles to ignite any fuel-air charge in front of the flame front and cause detonation. At these speeds, and at the increased charge temperatures and pressures in the combustion chamber of a turbocharged engine over that of a naturally aspirated unit, the resistance to detonation of the fuel typified by its octane number is therefore more significant.
So while the 2013 fuels may have been blended more for their rapid speed of burn, in 2014 the emphasis is more likely to be on the resistance of the fuel to detonation. And as each species of hydrocarbon – be it aromatic, olefin, paraffin or naphthene – will have its own speed of burn and blending octane, so the optimum fuel for each engine made up from varying amounts of these will almost certainly vary.
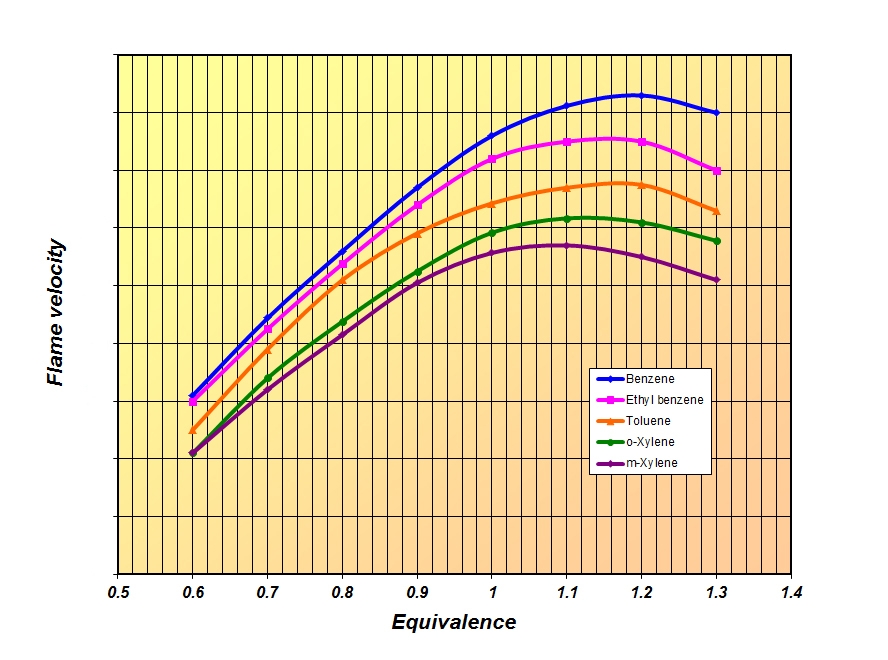 Fig. 1 - Comparative flame speeds of some commonly used aromatic hydrocarbons
Fig. 1 - Comparative flame speeds of some commonly used aromatic hydrocarbons
Written by John Coxon