Taking the heat
 In any internal combustion engine, the design or configuration of the cylinder or its liner is of critical concern. Exposed to the full effect of combustion at its upper part, which falls as we go down the cylinder, the component has to be as light as possible yet still retain its mechanical robustness with minimal distortion. As bmep (brake mean effective pressure) and engine speeds increase, cooling therefore assumes a major importance.
In any internal combustion engine, the design or configuration of the cylinder or its liner is of critical concern. Exposed to the full effect of combustion at its upper part, which falls as we go down the cylinder, the component has to be as light as possible yet still retain its mechanical robustness with minimal distortion. As bmep (brake mean effective pressure) and engine speeds increase, cooling therefore assumes a major importance.
Open-deck cylinder blocks, where the engine coolant is in close proximity to the liner top, are unpopular in race engines. Insufficient lateral location invariably creates cylinder head sealing issues. While ingenious solutions for increasing the wetted surface area have been used successfully, as heat fluxes increase then attention surely has to be paid to the cooling medium used, with the liner and heat transport fluid surrounding it being viewed as a system.
For most applications, water is the perfect heat transport fluid. Good specific heat characteristics, low viscosity - and, above all, its easy availability - have made it the most obvious candidate. Various additives will reduce its tendency to corrode while safeguarding against freezing and boosting its boiling temperature; ethylene glycol, for example, can be introduced generally at concentrations up to 50%. But the presence of water around the top of the liner can have both good and bad consequences.
In the cylinder head and around the top of the cylinder liner where large heat fluxes occur, the process of nucleate or instantaneous boiling next to the hot surfaces helps to conduct the heat into the body of the fluid. Heat is removed from the surface using the latent heat effect, and the fluid later condenses and offers up that heat when away from the liner wall. So long as there is a constant stream of coolant, the process will continue efficiently.
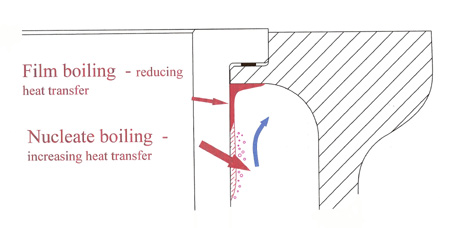
However, if the flow becomes stagnant for any reason, the steam produced will eventually act as a thermal barrier, resulting in local overheating. Known as film boiling, even if it doesn't harm the contact surface then the instantaneous pressure perturbations may cause fatigue damage and consequent pitting, eventually leading to liner failure. The presence of water in a high-performance cooling system, where heat fluxes are high and the flow uncertain, is surely therefore questionable.
Pressurising the system raises the boiling point of pure water to 121 C at 15 psig, but increasing the pressure brings an invisible cost - that of requiring better sealing, stronger hoses and a greater risk of cooling system failure at some time. The alternative of a 50% mix of ethylene glycol and water is only a partial cure because any boiling of water in the mix can still cause film boiling at critical parts. But since ethylene glycol is toxic, surely this is undesirable anyway.
One solution alternative to using ethylene glycol (C2H6O2) is to use a close cousin, propylene glycol (C3H8O2). It has a very similar boiling point, at around 180 C (at atmospheric pressure), and although the specific heat is around two-thirds that of water, when used as a heat transfer fluid it gives more consistent cooling at high heat flux and virtually eliminates film boiling.
Since propylene-based fluids have much greater viscosity, engines will have to be preheated, but since this is common practice anyway, it shouldn't cause any extra concern.
Above all, however, the non-toxic nature of propylene glycol means that any accidental spills are not particularly hazardous - and the workshop cat, useful in the keeping the mice under control, will be spared serious harm should it accidentally drink any.
Fig. 1 - Nucleate and film boiling
Written by John Coxon