Elixir of life
 It is often said that water is the elixir of life. But when used as a coolant in a high-performance racing engine it can be the kiss of death instead. Let me explain.
It is often said that water is the elixir of life. But when used as a coolant in a high-performance racing engine it can be the kiss of death instead. Let me explain.
Ever since the invention of the 'heat' engine, limits to the properties of the metals used mean they have necessitated some kind of cooling. And since water is all around us, it makes sense to use this is a transport fluid to remove heat from the cylinder head and disperse it into the atmosphere.
But water brings with it certain problems. When the temperature of the metal being cooled is lower than the boiling point of the transport fluid, the dissipation of heat is relatively straightforward, consisting mainly of conduction aided by convention in the fluid. So the greater the temperature difference the greater the heat flow.
As the heat flux rises, however, and the localised temperature of the surface exceeds the localised boiling point of the transport fluid, then a phenomenon known as nucleate boiling occurs at the boundary. This is good, and can actually increase the heat dissipation at that point.
A full account of the situation is quite complex, but essentially the peaks of the surface at a microscopic level intensify the heat flux and promote a change of state of the transport fluid from liquid to gas. At a very localised level, this process absorbs heat in the form of latent heat and makes the heat extraction process even more efficient. So long as the coolant flows quickly and is replaced by a fresh supply, the heat flows through the boundary at an increased rate. The gas thus created moves away from the surface and condenses again dispersing the heat into the bulk of the fluid.
But there may be times when these small bubbles of nucleate boiling are not transported away quite so easily, for instance when the heat flux is far greater or when the flow rate of coolant is restricted in some way. In such cases these bubbles will build up and form a continuous layer across the boundary, effectively insulating it in a 'blanket' of vapour. This can cause hot spots, erosion and possibly even detonation in extreme cases.
Compounds such as ethylene glycol that are added to coolant protect the water jacket under freezing conditions, and which are added at up to 50% of the total mixture, can raise the boiling point of the coolant slightly, as can pressurising the system. But when really high temperatures are generated by high levels of heat flux, the water in the system will have to be completely removed and replaced by something with a much higher boiling point.
In Advanced Gas Cooled nuclear reactors, for example, this cooling medium is pressurised carbon dioxide, although the inert gas helium has also been tried. For our purposes however, that's a bit excessive, even for the most extreme engine applications.
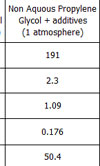
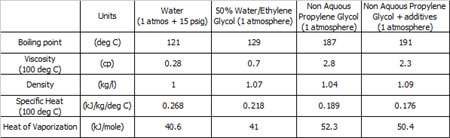
No, the way to go is to use propylene glycol, another of the non-aqueous glycol products sometimes found in industrial process plants. With a similar density to water, propylene glycol has a boiling point of 187º C and a latent heat from liquid to gas 30% greater than that of water.
This is offset though by a 30% reduction in its heat-bearing capacity (specific heat) compared to water and its high viscosity, particularly at low (about 10º C) ambient temperatures. Adding other components to form an homogenous mixture can mitigate this, to the detriment of some of propylene glycol's other properties, but as engineers we are well aware that in order to get one characteristic we may well have to compromise with another.
So, if water can be the 'kiss of death' for our racing engine, propylene glycol and its additives could be the 'elixir of life'.
Fig. 1 - Comparison of coolant properties
Written by John Coxon